
pharmaceutical validation services
Hvac validation equipment qualification process validation computer system validation 21 cfr part 11 compliance software validation activity quality control instrument validation plc validation scada system validation thermal validation temperature mapping sap erp validation bms ems validation spreadsheet validation quality system documentation (sop preparation) project qualifification network qualification it qualification compliance management clean room validation.
...more
Environment management system validation Services
Building management system (bms) and environment management system (ems) validation is required. As per gamp 5 all the computerized gxp system validation is required. Following documentation shall be provided during validation activity. User requirement specification initial risk assessment validation plan functional specification functional risk assessment installation qualification operation qualification performance qualification traceability matrix validation summary report.
...more
Spreadsheet validation
Spreadsheet validation is required for pharmaceutical industries. Now a days spreadsheet validation is essential. Following documents shall be provided during validation activity user requirement specification initial risk assessment validation plan functional specification functional risk assessment installation qualification operation qualification performance qualification traceability matrix validation summary report.
...more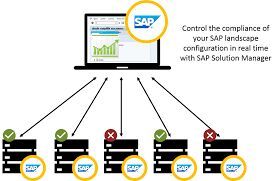
ERP Software
The aim of SAP ERP validation is to improve the quality systems. For sustained quality management, qualification and documentation are required in equal measure � regardless of whether we�re talking about operations, testing laboratories, the production process, or stockholding. Risks and weak points must be identified and averted.
...more
PLC Validation
Programmable logic control system validation. As per gamp 5 following document shall be provided during validation activity. User requirement specification initial risk assessment validation plan functional specification functional risk assessment installation qualification operation qualification performance qualification traceability matrix validation summary report.
...more
Computer System
We provide following documents as per gamp 5 requirement. initial risk assessment (ira) user requirement specification (urs) if requiredrn validation plan (vp) functional specification (fs) if requiredrn design specification (ds) functional risk assessment document (ra) installation qualification (iq) operational qualification (oq) traceability matrix (tm) validation summary report (vsr)
...moreBe first to Rate
Rate ThisOpening Hours