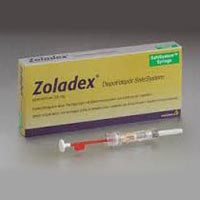
Zoladex Injection
ZOLADEX® (goserelin acetate implant) is a GnRH agonist. Goserelin acetate is chemically described as an acetate salt of [D-Ser(But)6,Azgly10]. Its chemical structure is pyro-Glu-His-Trp-Ser-Tyr-D-Ser(But)-Leu-ArgPro-Azgly-NH2 acetate [C59H84N18O14 •(C2H4O2)x where x = 1 to 2.4].Goserelin acetate is an off-white powder with a molecular weight of 1269 Daltons (free base). It is freely soluble in glacial acetic acid. It is soluble in water, 0.1M hydrochloric acid, 0.1M sodium hydroxide, dimethylformamide and dimethyl sulfoxide. Goserelin acetate is practically insoluble in acetone, chloroform and ether.ZOLADEX is supplied as a sterile, biodegradable product containing goserelin acetate equivalent to 3.6 mg of goserelin. ZOLADEX is designed for subcutaneous injection with continuous release over a 28-day period. Goserelin acetate is dispersed in a matrix of D,L-lactic and glycolic acids copolymer (13.3-14.3 mg/dose) containing less than 2.5% acetic acid and up to 12% goserelin-related substances and presented as a sterile, white to cream colored 1-mm diameter cylinder, preloaded in a special single use syringe with a 16-gauge x 36 +/-0.5 mm siliconized needle with protective needle sleeve (SafeSystem™ Syringe) in a sealed, light-and moisture-proof, aluminum foil laminate pouch containing a desiccant capsule. Studies of the D,L-lactic and glycolic acids copolymer have indicated that it is completely biodegradable and has no demonstrable antigenic potential.
...more
Wepox Injection
We hold expertise in providing a wide range of Wepox 4000 IU Injection (Rhu-erythropoietin). These are prepared in hygienic environment and with proper measure in formulating them. Our entire product range is treasured among them for its long shelf life and effective results.
...more
Velcade Injection
VELCADE® (bortezomib) for Injection is an antineoplastic agent available for intravenous injection or subcutaneous use. Each single use vial contains 3.5 mg of bortezomib as a sterile lyophilized powder. Inactive ingredient: 35 mg mannitol, USP.Bortezomib is a modified dipeptidyl boronic acid. The product is provided as a mannitol boronic ester which, in reconstituted form, consists of the mannitol ester in equilibrium with its hydrolysis product, the monomeric boronic acid. The drug substance exists in its cyclic anhydride form as a trimeric boroxine.The chemical name for bortezomib, the monomeric boronic acid, is [(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2[(pyrazinylcarbonyl) amino]propyl]amino]butyl] boronic acid.Bortezomib has the following chemical structure:VELCADE® (bortezomib) Structural Formula IllustrationThe molecular weight is 384.24. The molecular formula is C19H25BN4O4. The solubility of bortezomib, as the monomeric boronic acid, in water is 3.3 to 3.8 mg/mL in a pH range of 2 to 6.5.
Medicine Type : Allopathic
...more
Vaccines
An exhaustive range of Vaccines is provided by us in the International Market that has made us a major wholesale supplier & exporter in the healthcare industry. We are associated with many multinationals as well as WHO Prequalified Indian manufacturers. This means that our Vaccines are of superlative quality. Vaccines are usually needed on a large scale basis for immunization especially Vaccines for Polio and Hepatitis, that is why we always keep a bulk stock of Vaccines with us that is ready to be delivered when required. We offer Oral Polio VaccinesAnti Rabies Vaccines DPT VaccinesMeningitis VaccinesChicken Pox VaccinesInfluenza Vaccine MMR VaccinesHepatitis B VaccinesTD VaccinesTrivalent Vaccines, Quadrivalent Vaccines/ Pentavalent Vaccines etc. Why our Vaccines? Wide gamut of VaccinesMarket leading priceMinimum side effectsAvailable in bulk quantities
...more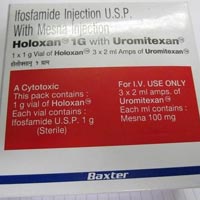
Uromitexan Injection
Uromitexan (mesna) New Zealand Data Sheet Uromitexan Data Sheet 7 August 2014 Page 1 of 14 Baxter UROMITEXAN Mesna 400mg tablets and 600mg film coated tablet Active ingredient : Mesna Inactive ingredients : calcium hydrogen phosphate, lactos e monohydrate, starch-maize, cellulose microcrystalline, povidone, magnesium stearate. Coating: hypromellose, Macrogol 6000, simethicone, titanium dioxide. Molecular formula : C2H5NaO3S2 with a molecular weight of 164.18. Structural formula : HS-CH2-CH2-SO3Na+ DESCRIPTION The active ingredient, mesna, is a synthetic sulphydryl compound designated as sodium 2- mercapto-ethane sulphonate. Uromitexan tablets are white oblong, biconvex film-coated tablets, scored on one side and marked “M 4” or “M 6”, containing 400mg or 600mg (respectively) of mesna.
Form : Liquid
...more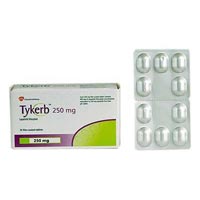
Tykerb Tablets
Lapatinib is a small molecule and a member of the 4-anilinoquinazoline class of kinase inhibitors. It is present as the monohydrate of the ditosylate salt, with chemical name N-(3- chloro-4-{[(3-fluorophenyl)methyl]oxy}phenyl)-6-[5-({[2- (methylsulfonyl)ethyl]amino}methyl)-2-furanyl]-4-quinazolinamine bis(4- methylbenzenesulfonate) monohydrate. It has the molecular formula C29H26ClFN4O4S (C7H8O3S)2 H2O and a molecular weight of 943.5. Lapatinib ditosylate monohydrate has the following chemical structure:TYKERB (lapatinib) Structural Formula IllustrationLapatinib is a yellow solid, and its solubility in water is 0.007 mg/mL and in 0.1N HCl is 0.001 mg/mL at 25°C.Each 250 mg tablet of TYKERB contains 405 mg of lapatinib ditosylate monohydrate, equivalent to 398 mg of lapatinib ditosylate or 250 mg lapatinib free base.The inactive ingredients of TYKERB are: Tablet Core: Magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate. Coating: Orange film-coat: FD&C yellow No. 6/sunset yellow FCF aluminum lake, hypromellose, macrogol/PEG 400, polysorbate 80, titanium dioxid
Packaging Type : Stripes
Application : Clinical, hospital etc.
...more
Temodal Capsules
TEMODAR contains temozolomide, an imidazotetrazine derivative. The chemical name of temozolomide is 3,4-dihydro-3methyl-4-oxoimidazo[5,1-d]-as-tetrazine-8-carboxamide. The structural formula is:TEMODAR® (temozolomide) Structural Formula IllustrationThe material is a white to light tan/light pink powder with a molecular formula of C 6H6N6O2 and a molecular weight of 194.15. The molecule is stable at acidic pH ( < 5) and labile at pH > 7; hence TEMODAR can be administered orally and intravenously. The prodrug, temozolomide, is rapidly hydrolyzed to the active 5-(3-methyltriazen-1-yl) imidazole-4-carboxamide (MTIC) at neutral and alkaline pH values, with hydrolysis taking place even faster at alkaline pH.TEMODAR Capsules : Each capsule for oral use contains either 5 mg, 20 mg, 100 mg, 140 mg, 180 mg, or 250 mg of temozolomide.The inactive ingredients for TEMODAR Capsules are as follows : TEMODAR 5 mg : lactose anhydrous (132.8 mg), colloidal silicon dioxide (0.2 mg), sodium starch glycolate (7.5 mg), tartaric acid (1.5 mg), and stearic acid (3 mg). TEMODAR 20 mg : lactose anhydrous (182.2 mg), colloidal silicon dioxide (0.2 mg), sodium starch glycolate (11 mg), tartaric acid (2.2 mg), and stearic acid (4.4 mg). TEMODAR 100 mg : lactose anhydrous (175.7 mg), colloidal silicon dioxide (0.3 mg), sodium starch glycolate (15 mg), tartaric acid (3 mg), and stearic acid (6 mg). TEMODAR 140 mg : lactose anhydrous (246 mg), colloidal silicon dioxide (0.4 mg), sodium starch glycolate (21 mg), tartaric acid (4.2 mg), and stearic acid (8.4 mg). TEMODAR 180 mg : lactose anhydrous (316.3 mg), colloidal silicon dioxide (0.5 mg), sodium starch glycolate (27 mg), tartaric acid (5.4 mg), and stearic acid (10.8 mg). TEMODAR 250 mg : lactose anhydrous (154.3 mg), colloidal silicon dioxide (0.7 mg), sodium starch glycolate (22.5 mg), tartaric acid (9 mg), and stearic acid (13.5 mg). The body of the capsules is made of gelatin, and is opaque white. The cap is also made of gelatin, and the colors vary based on the dosage strength. The capsule body and cap are imprinted with pharmaceutical branding ink, which contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, purified water, strong ammonia solution, potassium hydroxide, and ferric oxide. TEMODAR 5 mg : The green cap contains gelatin, titanium dioxide, iron oxide yellow, sodium lauryl sulfate, and FD&C Blue #2. TEMODAR 20 mg : The yellow cap contains gelatin, sodium lauryl sulfate, and iron oxide yellow. TEMODAR 100 mg : The pink cap contains gelatin, titanium dioxide, sodium lauryl sulfate, and iron oxide red. TEMODAR 140 mg : The blue cap contains gelatin, sodium lauryl sulfate, and FD&C Blue #2. TEMODAR 180 mg : The orange cap contains gelatin, iron oxide red, iron oxide yellow, titanium dioxide, and sodium lauryl sulfate. TEMODAR 250 mg : The white cap contains gelatin, titanium dioxide, and sodium lauryl sulfate. TEMODAR for Injection : Each vial contains 100 mg of sterile and pyrogen-free temozolomide lyophilized powder for intravenous injection. The inactive ingredients are : Mannitol (600 mg), L-threonine (160 mg), polysorbate 80 (120 mg), sodium citrate dihydrate (235 mg) and hydrochloric acid (160 mg).
Strength : 100mg
...more
Taxotere Injection
Application : Breast, Lung, Prostate, Stomach, and Head and Neck Cancer
Taxol Injection
TAXOL (paclitaxel) Injection is a clear, colorless to slightly yellow viscous solution. It is supplied as a nonaqueous solution intended for dilution with a suitable parenteral fluid prior to intravenous infusion. TAXOL is available in 30 mg (5 mL), 100 mg (16.7 mL), and 300 mg (50 mL) multidose vials. Each mL of sterile nonpyrogenic solution contains 6 mg paclitaxel, 527 mg of purified Cremophor® EL (polyoxyethylated castor oil) and 49.7% (v/v) dehydrated alcohol, USP.Paclitaxel is a natural product with antitumor activity. TAXOL (paclitaxel) is obtained via a semi-synthetic process from Taxus baccata. The chemical name for paclitaxel is 5β,20-Epoxy-l,2α,4,7β,10β,13α-hexahydroxytax-l l-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine.Paclitaxel has the following structural formula:TAXOL® (paclitaxel) INJECTION Structural Formula IllustrationPaclitaxel is a white to off-white crystalline powder with the empirical formula C47H51NO14 and a molecular weight of 853.9. It is highly lipophilic, insoluble in water, and melts at around 216-217° C.
...more
Rhogam Injection
Rhogam Injection
Type : Allopathic medicine
Quadri Meningo Vaccines
Menomune® A/C/Y/W-135, Meningococcal Polysaccharide Vaccine, Groups A, C, Y and W-135 Combined, for subcutaneous use, is a freeze-dried preparation of the group-specific polysaccharide antigens from Neisseria meningitidis, Group A, Group C, Group Y and Group W-135. N meningitidis are cultivated with Mueller Hinton agar 1 and Watson Scherp 2 media. The purified polysaccharide is extracted from the Neisseria meningitidis cells and separated from the media by procedures which include centrifugation, detergent precipitation, alcohol precipitation, solvent or organic extraction and diafiltration. No preservative is added during manufacture. The 0.78 mL vial of diluent contains sterile, preservative-free, pyrogen-free distilled water and is used for reconstitution of product supplied in 1 mL vials. The 6 mL vial of diluent contains sterile, pyrogen-free distilled water to which thimerosal (mercury derivative) 1:10,000 is added as a preservative. The 6 mL vial is for reconstitution of product supplied in 10 mL vials. After reconstitution with diluent as indicated on the label, the 0.5 mL dose is formulated to contain 50 μg of “isolated product” from each of Groups A, C, Y and W-135 in an isotonic sodium chloride solution. Each dose of vaccine is also formulated to contain 2.5 mg to 5 mg of lactose added as a stabilizer. 3 The vaccine when reconstituted is a clear colorless liquid. Potency is evaluated by measuring the molecular size of each polysaccharide component using a column chromatography method as standardized by the US Food and Drug Administration (FDA) and the World Health Organization (WHO) 4 for Meningococcal Polysaccharide Vaccine. THIS VACCINE CONFORMS TO THE WORLD HEALTH ORGANIZATION (WHO) REQUIREMENTS
Usage : Clinical, Hospital
Purity : 100%
Form : Vaccines
Packaging Type : Bottle
Storage : Store at 20o to 8oC
...more
Proleukin Injection
Nterleukin-2 (aldesleukin, [Proleukin®]) is a cytokine with the following immune - modulating effects: enhancement of lymphocyte mitogenesis and cytotoxicity, induction of lymphocyte - activated (LAK) cells and natural killer (NK) cells, and induction of interferon gamma production. U.S. Food and Drug Administration (FDA) - approved labeled indications include the treatment of metastatic renal cell carcinoma and metastatic malignant melanoma. Other oncologic applications of IL - 2 monotherapy are being actively investigated, principally as a technique to maintain remission or eliminate minimal residual disease in patients with a variety of malignancies, including most prominently leukemias, but also including lymphomas and other solid tumors such as neuroblastoma and breast cancer. IL - 2 has also been used post - autologous bone marrow transplantation as an immunotherapeutic technique to maintain remission and reduce the relapse rate. In HIV-infected patients, there is a reduced endogenous production of IL-2 and a defect in IL-2 receptor expression, respon sible in part for the characteristic reduction in CD4 counts. Exogenous IL-2, in conjunction with antiretroviral therapy, has been investigated as a technique to increase CD4 counts, preserve immune function and hopefully decrease the incidence of opportu nistic infections. It has been proposed that IL-2 in conjunction with combination antiretroviral therapy may be a useful approach for purging HIV from the latently infected CD4 cells. IL-2 has also been investigated as a component of a variety of combinat ion therapies, for example in combination with interferon alpha in the treatment of metastatic renal cell cancer and metastatic melanoma. IL -2 can be administered by subcutaneous injection (low-dose therapy) or into a vein (intermediate or high-dose therapy), either intermittently as a bolus or quick injection, or as a continuous treatment over a defined period of time.
Packaging Type : Bottle
Form : Injection
Storage : Cool and Dry Place
Application : Clinical, hospital etc.
Dosage : Directed by the physician
...more
Primacor Vaccines
PRIMACOR™ 3003 Copolymer is an ethylene acrylic acid copolymer which has been specifically designed by Dow for use as an adhesive or sealant layer in extrusion coating and extrusion lamination.PRIMACOR 3003 Copolymer exhibits : Excellent draw-down and edge stability Excellent organoleptic properties Excellent toughness and strength Outstanding environmental stress crack and product resistance Excellent hot-tack and sealability Adhesion to paper, paperboard, metals and polyethylenes Insensitivity to moisture
Type : Vaccines
Application : Clinical, Hospital
Purity : 100%
Form : Liquid
Packaging Type : Bottle
...more
Pharmaceutical Syrups
As a renowned Exporter, we bring forth a wide variety of Pharmaceutical Syrups which are used for curing various ailments. These Pharmaceutical Syrups include syrups for treating ailments like cough, vomiting, etc. Our range of Pharmaceutical Syrups is available in tamper-proof packaging so as to ensure zero contamination. Further, our Pharmaceutical Syrups are also valued for their accurate composition, purity, long shelf life and zero side effects. Owing to these features, our Pharmaceutical Syrups are widely demanded by pharmaceutical industry.We offer the following Pharmaceutical Syrups : Cristarox dry syrup : Each 5ml after reconstitution consists of cefuroxime axetil u. S. P equivalent to cefuroxime 125 mg Vomitron syrup : Each 5ml contains ondansetron dihydrochloride equivalent to ondansetron u. S. P 2mg. Its colors are sunset yellow and carmoisine Cristaclor oral suspension : Each 5 ml after reconstitution contains cefaclor u. S. P: 125 mg/5 ml Curox 30 ml oral suspensions : Each 5ml contains ofloxacin u. S. P 50mg in flavored syrup
Shelf Life : 1year
Purity : 100%
Form : Liquid
...more
Pharmaceutical Capsules
We are engaged in the business of exporting highly effective Pharmaceutical Capsules, which are procured from the most reliable sources. Prepared using the accurate combination of different chemicals, these Pharmaceutical Capsules are widely recommended by renowned medical practitioners. These Pharmaceutical Capsules are widely used for the treatment of various ailments so as to provide relief to the patients.Pharmaceutical Capsules are widely used for medical purpose in the pharmaceutical industry. Various Pharmaceutical Capsules, offered by us, are mentioned below:Cristaclor 250 capsules : Cefaclor USP 250 mg Cristaclor 500 capsules : Cefaclor USP 500 mg Zozibact 250 capsules : Azithromycin USP 250 mg
Form : Capsules
Medicine Type : Allopathic
...more
Paclitaxel Protein Bound Injection
Paclitaxel protein-bound injection is used to treat metastatic breast cancer (cancer that has already spread) after other treatments have failed. It is used together with carboplatin, a cancer medicine, to treat advanced or metastatic non-small cell lung cancer in patients who cannot receive radiation therapy or have surgery. Paclitaxel protein-bound is used together with gemcitabine, a cancer medicine, to treat metastatic pancreas cancer.Paclitaxel belongs to the group of medicines called antineoplastics (cancer medicines). It interferes with the growth of cancer cells, which are eventually destroyed by the body. Since the growth of normal body cells may also be affected by paclitaxel, other unwanted effects will also occur.This medicine is to be given only by or under the direct supervision of a doctor.
Form : Liquid
Usage : Clinical, hospital etc.
Packaging Type : Bottle
Storage : Cool and Dry Place
Form : Liquid
...more
Oral Polio Vaccine
We take immense pleasure in introducing ourselves as one of the leading companies engaged in exporting of Oral Polio Vaccine. Oral Polio Vaccine is a mixture of live attenuated poliovirus strains of three stereotypes, selected by their ability to mimic the immune response following infection with wild polioviruses. Oral Polio Vaccine produces antibodies in the blood of all three types of poliovirus and in the event of infection it protects against polio paralysis by preventing the spread of poliovirus to the nervous system. Oral Polio Vaccine that we provide does not have any side effects.
Packaging Size : 20 Dose
Packaging Type : Tube
Usage : Clinical,Hospital
Form : Liquid
...more
oncology products
We deal in Oncology Medicines to provide solutions for a healthy life. Our Oncology Drugs are provided in a variety of strengths & different packaging specifications as per the needs of client and end users. Our Oncology Drugs are really revolutionary in terms of the research that goes into them. We offer Oncology Drugs to fight different forms of cancer and they are widely used by doctors at the international arena. We never compromise on the quality or packaging making it easier to safely transport the Oncology drugs all over the world. We maintain strict standards while delivering the Oncology Drugs within the stipulated timeframes. Our Range of Oncology Drugs Oncology MedicinesOvarian Cancer DrugColorectal Cancer DrugProstate Cancer DrugAnti Cancer MedicineOncology ProductsLung Cancer DrugSkin Cancer DrugBreast Cancer Drugs etc. Availability TabletsCapsulesInjectionsVials/Ampoules Our Oncology Drugs are famous for Availability in a broad rangeSafe packaging to ensure easy transportHighly effectiveLow side effectsAffordable prices Products under Development Erlotinib TabletsTrastazumab InjectionRituximab InjectionInterferon Alpha / Beta / GammaLipid Complex - DoxorubucinSorafenib Tablets
...more
Nolvadex Tablets
What has caused enormous wave of buying prescribed medicine from Canada? The simple answer - Internet. A customers’ revolution of sorts (of knowledge and buying power) has been created as a matter of fact that people all over the world could choose the best prices of any product they need just sitting an comfort and convenience at home.No more people need up to overpay for vital products such as prescribed medications. The saying “Knowledge is a power” is well-known. What does it mean in the world of prescribed medication? People who once knew about paying less for the same drugs using Canadian prescribed orders-will never agree to pay huge prices for their drugs. It’s easy to get the cheapest Canadian prescribed drugs if you have internet connection and doctor’s agreement. The days of waiting in a queue in your local drugstore to fill the recipe are gone. The days of paying enormous sums of money for the vital drugs are also gone.Nowadays you can find exactly all you need quickly and easily. That’s happen thanks to the explosion of reduced online prescribed medicaments. It’s good to mention that you can now do your online prescribe medicine order sitting in comfort and privacy of your own home! Your personal deal will be saved strictly in security by the network of pharmacy professionals, who control your transactions.You just research internet a little bit and the cheapest Canadian prescribed drugs can be yours. There are many Canadian online drugstores, but be careful and don’t trust all! Work hard and check if they have got a license, one pharmacist on hand at least to fill your order, check the quality of guaranty, speed of delivery and security.All internet pharmaceutical drugstores must be easy to use, offer full information about the cheapest Canadian medicines they propose. They should contain price comparisons, what will help you to see how many savings you can get on their products. Their toll-free phone number gives u a possibility to call them all time you need, When you dial this number, you must have a possibility to talk with an expert live, discuss with him other questions you have.
Type : Finished Product
Packaging Size : 3x10
Packaging Type : Strip
...more
Nexavar Tablets
Sorafenib tosylate is a white to yellowish or brownish solid with a molecular weight of 637g/mol. Sorafenib tosylate is practically insoluble in aqueous media, slightly soluble in ethanol and soluble in PEG 400. In additio n to sorafenib tosylate, each NEXAVAR tablet contains the following inactive ingredients : croscarmellose sodium microcrystalline cellulose hypromellose sodium lauryl sulphate magnesium stearate macrogol titanium dioxide iron oxide red
Strength : 200mg
...more
Neupogen Injection
Filgrastim is a human granulocyte colony-stimulating factor (G-CSF)‚ produced by recombinant DNA technology. NEUPOGEN® is the Amgen Inc. trademark for Filgrastim‚ which has been selected as the name for recombinant methionyl human granulocyte colony-stimulating factor (r-metHuG-CSF).NEUPOGEN® is a 175 amino acid protein manufactured by recombinant DNA technology.1 NEUPOGEN® is produced by Escherichia coli (E coli) bacteria into which has been inserted the human granulocyte colony-stimulating factor gene. NEUPOGEN® has a molecular weight of 18‚800 daltons. The protein has an amino acid sequence that is identical to the natural sequence predicted from human DNA sequence analysis‚ except for the addition of an N-terminal methionine necessary for expression in E coli. Because NEUPOGEN® is produced in E coli‚ the product is nonglycosylated and thus differs from G-CSF isolated from a human cell.NEUPOGEN® is a sterile‚ clear‚ colorless‚ preservative-free liquid for parenteral administration containing Filgrastim at a specific activity of 1.0 ± 0.6 x 108 U/mg (as measured by a cell mitogenesis assay). The product is available in single use vials and prefilled syringes. The single use vials contain either 300 mcg or 480 mcg Filgrastim at a fill volume of 1.0 mL or 1.6 mL, respectively. The single use prefilled syringes contain either 300 mcg or 480 mcg Filgrastim at a fill volume of 0.5 mL or 0.8 mL, respectively. See table below for product composition of each single use vial or prefilled syringe. 300 mcg/ 1.0 mL Vial 480 mcg/ 1.6 mL Vial 300 mcg/ 0.5 mL Syringe 480 mcg/ 0.8 mL SyringeFilgrastim 300 mcg 480 mcg 300 mcg 480 mcgAcetate 0.59 mg 0.94 mg 0.295 mg 0.472 mgSorbitol 50.0 mg 80.0 mg 25.0 mg 40.0 mgPolysorbate 80 0.04 mg 0.064 mg 0.02 mg 0.032 mgSodium 0.035 mg 0.056 mg 0.0175 mg 0.028 mgWater for Injection USP q.s. ad 1.0 mL 1.6 mL 0.5 mL 0.8 mLREFERENCES1. Zsebo KM‚ Cohen AM‚ Murdock DC‚ et al. Recombinant human granulocyte colonystimulating factor: Molecular and biological characterization. Immunobiol. 1986;172:175-184.
...more
Neulastim Injection
Neulastim® DS 1402051 Data Sheet Neulastim® Pegfilgrastim 6 mg in 0.6 mL (pre-filled syringe), solution for injection Description Therapeutic/Pharmacologic Class of Medicine Haematopoietic growth factor Pharmacotherapeutic group: Cytokines, ATC Code: L03AA13 Type of Dosage Form Solution for injection in a pre-filled syringe. Route of Administration Subcutaneous injection. Sterile/Radioactive Statement Sterile. Qualitative and Quantitative Composition 6 mg of pegfilgrastim in 0.6 mL (10 mg/mL*) solution for injection. * Based on protein only. The concentration is 20 mg/mL if the PEG moiety is included. Pegfilgrastim is composed of filgrastim (recombinant methionyl human G-CSF) with a 20 kDa polyethylene glycol (PEG) molecule covalently bound to the N-terminal methionine residue. Filgrastim is produced by recombinant DNA technology in E coli (K12). Excipients: Sodium acetate**, Sorbitol, Polysorbate 20, Water for injections ** Sodium acetate is formed by titrating glacial acetic acid with sodium hydroxide
...more
Neotrexate Tablets
Packaging Size : 10 x 10 Tablets
Packaging Type : Stripes
Mmr Vaccine
We are one of the leading Exporters of MMR Vaccine that acts as an immunization vaccine against life-threatening diseases like mumps, measles and rubella. Our MMR Vaccine is provided to children around one year and another dose of it is administered at the age of 4-5 years. We provide MMR Vaccine of well-known multinational companies like Merc &Co., GlaxoSmithKline Biologicals, and Sanofi Pasteur with the brand names of M-M-R II, Priorix, and Trimovax respectively. Long-term effects and efficacy are subject to continuing study.
Usage : Clinical
Packaging Size : 10 vials in one box
Packaging Type : Vial
Form : Liquid
...moreOpening Hours