
Automatic Liquid Process Plant
Get Price Quote
We are offering automatic liquid process plant. Bullet moc ss 304 316 316l. Bullet we can manufacture as per customer design requirement. Bullet electric heating also possible for smaller capacity. Bullet top mounting stirrer homogenizer also available. Bullet plc based fully automatic plant also available. Bullet cgmp with documentation

CIP Tank
Get Price Quote
Online parameters Completely automated process cycle Online PH conductivity,TOC sensing Single to multitank system
Best Deals from Milk Processing Plant and Machines
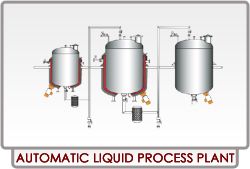
Automatic Liquid Process Plant
Get Price Quote
As, our organization has stringent quality management strategies, our complete range of products is preferred by many across the industry. Clients can select any payment mode from our online or offline methods. Both of them are secure, hassle free, and ensure the fastest trade with the customers.Details : MOC SS 304 / 316 / 316L. We can Manufacture as per customer design / requirement. Electric heating also possible for smaller capacity. Top mounting stirrer / homogenizer also available. PLC based Fully automatic plant also available. cGMP with documentation

Cip System
180,000 Per Piece
CIP is commonly used for cleaning bioreactors, fermenters, mix vessels, and other equipment used in biotech manufacturing, pharmaceutical manufacturing and food and beverage manufacturing. CIP is performed to remove or obliterate previous Cell Culture batch components. It is used to remove in-process residues, control bioburden, and reduce endotoxin levels within processing equipment and systems. Residue removal is accomplished during CIP with a combination of heat, chemical action, and turbulent flow. The Code of Federal Regulations states “Equipment and utensils shall be cleaned, maintained, and sanitized at appropriate intervals to prevent malfunctions or contamination that would alter the safety, identity, strength, quality or purity of the drug product beyond the official or other established requirements.”[1] Repeatable, reliable, and effective cleaning is of the utmost importance in a manufacturing facility. Cleaning procedures are validated to demonstrate that they are effective, reproducible, and under control. In order to adequately clean processing equipment, the equipment must be designed with smooth stainless steel surfaces and interconnecting piping that has cleanable joints. The chemical properties of the cleaning agents must properly interact with the chemical and physical properties of the residues being removed. A typical CIP cycle consists of many steps which often include (in order): Pre-rinse with WFI (water for injection) or PW (purified water) which is performed to wet the interior surface of the tank and remove residue. It also provides a non-chemical pressure test of the CIP flow path. Caustic solution single pass flush through the vessel to drain. Caustic is the main cleaning solution. Caustic solution re-circulation through the vessel. Intermediate WFI or PW rinse Acid solution wash – used to remove mineral precipitates and protein residues. Final rinse with WFI or PW – rinses to flush out residual cleaning agents. Final air blow – used to remove moisture remaining after CIP cycle. Critical parameters must be met and remain within the specification for the duration of the cycle. If the specification is not reached or maintained, cleaning will not be ensured and will have to be repeated. Critical parameters include temperature, flow rate/supply pressure, chemical concentration, chemical contact time, and final rinse conductivity (which shows that all cleaning chemicals have been removed).

Pressure Gauge
500 Per Piece

SIP System
Get Price Quote
Are you searching for Wholesale SIP System? If yes, then you are at right place. Our company is identified as one of the proficient Manufacturers, Exporters and Suppliers of SIP System. Our production team makes use of high quality metal components and sophisticated technology in compliance with set industrial standards and norms. This system is highly used in pharmaceutical industries, biotechnology industries and food & beverage industries. Scope of Mobile SIP Unit & Features : Pure Steam Header with PI, TI & Safety Valve Condensate Header with sterile steam traps unit and Temp. Sensor PLC Control Panel with Time / Temperature control program, printer, alarm etc. as required SS Mobile Stand with PU Castors Features encompassed with the system like meeting designing to cGMP criteria; orbital welding; automation as per MCA & USFDA 21 CFR Part 11 with 100% drain ability design criteria. Documented output of cleaning & sterilization cycles. The CIP System controls the cleaning “T.A.C.T” parameters of Temperature, Action (velocity / pressure), Chemical concentration and Time of exposure. Application : Process Equipments for Industries Pharmaceutical Biotechnology Food Beverage

Pressure Gauge
Get Price Quote
Pressure Gauge, Stainless Steel Sheet, thermoware, ferrous utensils

Automatic C.I.P. Systems
Get Price Quote
Automatic C.I.P. Systems, uv water treatment system

Milk Chilling Plant
Get Price Quote
Milk Chilling Plant, Air Cooled Chillers, Ultra Low Temperature Deep Freezer

Pressure Gauge
Get Price Quote
Pressure Gauge, actuator valve, Silicone, Rubber Chemicals, Polyurethane